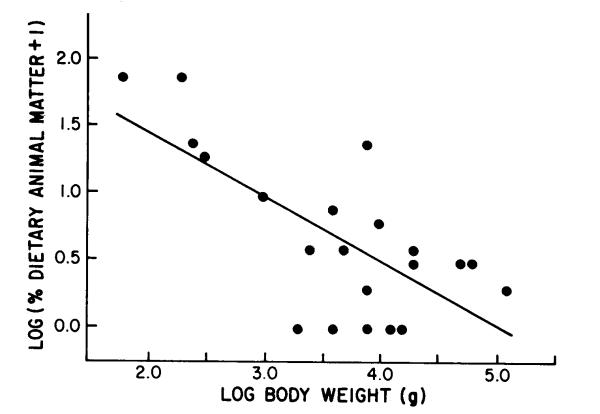
Fig. 1. Log-log regression of mean percent dietary animal matter vs. body weight for 21 nonhuman primates.
BioScience, Volume 28, Pages 761-766, 1978
Copyright © 1978 American Institute of Biological Sciences. Reproduced at www.curtbusse.com with permission of the American Institute of Biological Sciences via the Copyright Clearance Center.
Primate Carnivory and
Its Significance to Human Diets
W. J. Hamilton III and C. D. Busse
Recent studies of primate ecology and feeding behavior have expanded our knowledge of the diet of free-ranging primates (summarized in Clutton-Brock and Harvey 1977), especially concerning the degree to which primates consume animal prey. Many primate species once considered herbivorous are now known to expand the animal-matter portion of their diet to high levels when it is possible to do so.
Here we describe patterns of animal-matter consumption by primates and identify phylogenetic, ecological, and behavioral principles of carnivory applicable to primates and possibly to omnivores in general. This analysis may enhance understanding of the meat over-consumption phenomenon typical of contemporary luxury human diets.
Carnivory is used broadly here to refer to capture and ingestion of invertebrates and vertebrates regardless of size. Thus, we use the term to identify diet rather than behavior. Since foraging tactics of primates have considerable significance to the overall understanding of primate biology, these tactics are also considered here.
Most primates secure animal matter by close-range detection of prey items and capture without chase. Such scavenge-hunting (Hamilton 1973) requires no special predatory skills and is characteristic of omnivorous mammals, including subsistence human hunters. Active hunting, during which prey are actively stalked (Hamilton 1973), is a less common predatory tactic for contemporary primates. Stalking hunting, widely used by diverse predators to secure larger and fleeter prey, so far has been reported for primates only on limited occasions for chimpanzees hunting baboons (Papio anubis (Teleki 1973, Wrangham 1975) and for one population of baboons (P. anubis) hunting infant gazelles (Strum 1975).
Insect food is the predominant animal matter resource for primates. Insects are eaten by all extant apes, i.e., chimpanzees (e.g., Lawick-Goodall 1968), orang-utans (Gladikas-Brindamour1), gorillas (Fossey2), gibbons (Chivers 1972, R. L. Tilson3), and the siamang (Chivers 1972), and by most monkeys and prosimians. The amount of insect matter in most primate diets is small, but may expand to more than 90% of the diet when insects are abundant and easily captured. Since palatable and accessible prey species often occur only seasonally (Hamilton et al. 1978, Hausfater 1976), the amount of animal matter in primate diets can change dramatically throughout the year.
Two primate groups, the Colobinae, a group of Old World monkeys, and the Indriidae, a group of large prosimians, are folivorous and anatomically specialized to digest plant fiber (Bauchop 1971, Bauchop and Martucci 1968, Hill and Rewell 1948). These primates may incidentally ingest some insect matter with their staple plant foods, but among them only red colobus monkeys (Struhsaker 1975) and langurs (Yoshiba 1968) are reported to hunt insects actively. Except for these specific phyletic divergences towards herbivory, most primates expand the amount of animal matter in the diet when it is economically possible to do so.
We observed the facultative trend toward increased carnivory during field studies of chacma baboons, Papio ursinus, in the Namib Desert, Namibia, and for another population of the same species in the Okavango Swamp, Botswana (Hamilton et al. 1978). In the Namib, an outbreak of grasshoppers led to a nearly complete dietary shift to insectivory. As a result of this dietary shift to insects, these troops virtually eliminated daily treks through their home ranges. Troops settled near favored sleeping cliffs and permanent water holes and remained there for the duration of the grasshopper outbreak.
In the Okavango Swamp we observed a similar situation in the summer of 1973. An enormous outbreak of scale insects (Homoptera; Coccidae) on mopane trees occurred in the ranges of several troops. For troops whose home range included mopane trees, this outbreak led to a dietary shift emphasizing these insects. Seventy-two percent of all time allocated to feeding went to scale insect procurement. Adjacent troops, without this insect food resource, maintained a nearly exclusively vegetarian diet during the same interval (Hamilton et al. 1978). The evidence for choice is particularly convincing because troops with and without the mopane scale insect resource had the same additional alternative food options. Preference for animal matter seems confirmed.
Less commonly, primates, especially baboons and chimpanzees, kill larger prey, ranging in size from rodents to infant antelopes and the adult females of other primate species (e.g., Lawick-Goodall 1968, Nishida 1968, Suzuki 1971). Cooperative hunting by primates
_____________________
The authors are with the Institute of Ecology, University of California, Davis, CA 95616. © 1978 American Institute of Biological Sciences. All rights reserved.
_____________________
1. B. Gladikas-Brindamour, Anthropology Department, University of California, Los Angeles, personal communication, 1976.
2 D. Fossey, Karisoke Research Centre, Ruhengeri, Rwanda, personal communication, 1976.
3 R. L. Tilson, Namib Desert Research Station, Walvis Bay, Namibia, personal communication, 1977.
has been reported less frequently (Strum 1975, Teleki 1973).
BODY SIZE RELATIONSHIPS
Average animal-matter consumption for 21 primate species is tabulated in Table 1. The relative proportion of animal matter in primate diets has so far been determined by time budgets and stomach content analyses, rather than by the more desirable dry weight basis. Many of these time-budget values are underestimates of the extent of animal-matter consumption because feeding behavior is often difficult to observe, especially for arboreal primates, and insect consumption can be easily overlooked. Nevertheless, methodological standardization should not change the overall nature of the observed trends.
TABLE 1. Average body weight and percent dietary animal matter for 21 nonhuman primate species.*
| Species | Body wt. (g) |
Animal matter(%) |
Sources |
|---|---|---|---|
| Galago demidovii | 65 | 70 (s) | Charles-Dominique 1974 |
| Arctocebus colabarensis | 200 | 85 (s) | Charles-Dominique 1974 |
| Galago alleni | 260 | 25 (s) | Charles-Dominique 1974 |
| Galago elegantulus | 300 | 20 (s) | Charles-Dominique 1974 |
| Perodicticus potto | 1,100 | 10 (s) | Charles-Dominique 1974 |
| Lemur catta | 2,103 | 0 | Jolly 1966, Napier & Napier 1967 |
| Cercocebus galeritus | 2,559 | 3 (s) | Quris 1975 |
| Propithecus verreauxi | 3,600 | 0 | Richard 1974, Clutton-Brock & Harvey 1977 |
| Cercopithecus aethiops | 4,000 | 0 | Dunbar & Dunbar 1974, Hill 1970 |
| Cercopithecus nictitans | 5,232 | 3 (s) | Gautier-Hion & Gautier 1974, Hill 1970 |
| Presbytis senex | 8,172 | 0 | Hladik 1977, Napier & Napier 1967 |
| Ateles geoffroyi | 8,345 | 1 | J. Cant†, Klein & Klein 1977, Crile & Quiring 1940 |
| Cercocebus albigena | 8,875 | 24 | Waser 1975, Hill 1970 |
| Symphalangus syndactylus | 10,700 | 5 | Chivers et al. 1975, Napier_& Napier 1967 |
| Presbytis entellus | 12,826 | Trace | Hladik 1977, Napier & Napier 1967 |
| Theropithecus gelada | 17,060 | Trace | Dunbar & Dunbar 1974, Dunbar 1977, Napier & Napier 1967 |
| Papio anubis | 18,000 | 3 | Dunbar & Dunbar 1974, Napier & Napier 1967 |
| Papio ursinus | 20,600 | 2 | Hamilton et al. 1978, Hill 1970 |
| Pan troglodytes | 44,750 | 2 | Wrangham 1975, Napier & Napier 1967 |
| Pongo pygmaeus | 58,103 | 2 | Rodman 1977, MacKinnon 1974, Schultz 1941 |
| Gorilla gorilla | 126,250 | 1 | Fossey‡, Napier & Napier 1967 |
*"s" indicates data obtained from stomach and caecum content samples. Other determinations are based on time budgets and time samples. Data for each species are based on at least 10 stomach samples or 100 hours of field observation. The last reference for each species is the body weight source.
†J. Cant. Department of Anthropology. Univ. Colorado, Colorado Springs, personal communication, 1971.
‡D. Fossey, Karisoke Research Cantre. Ruhengeri, Rwanda, personal communication, 1976.
Smaller primate species generally consume proportionally more animal matter than do larger ones. A few small prosimians are almost completely insectivorous. Summarized estimates show that the amount of animal-matter consumption by primate species is inversely related to body weight (Fig. 1). A linear regression of log mean animal-matter consumption against log body weight for 21 nonhuman primate species (r =.0.70) shows a slope significantly different from zero (t.99, 19 = -3.95, p < .01).

Fig. 1. Log-log regression of mean percent dietary animal matter vs. body weight for 21 nonhuman primates.
Primates encompass so little variation in morphological adaptation to carnivory that trends towards or from specialization associated with body size differences are dimly distinguishable. The species most morphologically specialized towards carnivory is the aye-aye, Daubeutonia, a small (approx. 2.8 kg) lemur with an enormously elongated third finger which it uses to pry insects from under bark.
At the large end of the size spectrum, including the ground-dwelling primates, expansion of dietary animal-matter intake is based on facultative foraging responses to temporary pulses in animal-matter availability and to the implementation of new traditions, including the elaboration of new hunting tactics (Strum 1975). The social groups of ground-dwelling primates give them a high degree of protection from predators. This enhances their potential to utilize small food items because individuals can spend long intervals feeding without maintaining a high predator alert (Hamilton et al. 1978).
HYPOTHESES
Several hypotheses attempt to explain the primate preference for animal matter.
Non-Nutritional Hypotheses
These hypotheses suggest that predation, especially by chimpanzees upon baboons, does not have a nutritional basis. Kortlandt (1972) believes that chimpanzee predation upon baboons reduces interspecific competition for food. There is broad overlap of chimpanzee (Teleki 1975, Wrangham 1975) and baboon (Papio anubis) diets, but some preferred animal prey species for chimpanzees, such as red colobus monkeys (Busse 1977, Lawick-Goodall 1968, Wrangham 1975), an arboreal folivore, have little dietary overlap with chimpanzees (Clutton-Brock 1975). Baboons (P. anubus, P. cynocephalus) regularly capture neonate gazelles (Harding and Strum 1976, Hausfater 1976), another noncompeting prey species. For the
most part the insect prey of baboons and other primates are not direct competitors for food. Thus Kortlandt's interspecies exclusion hypothesis is unsupported as an exclusive explanation for why primates kill prey.
Kortlandt (1972) has suggested that capture of large prey such as baboons by chimpanzees may be aggression redirected from conspecifics towards prey. This hypothesis is based upon observations of hunting episodes by groups of chimpanzees near the Gombe Research Station headquarters in Tanzania. Hunting there probably was induced by exceptional concentrations of chimpanzees and baboons in response to banana provisioning. Subsequent observations under less modified conditions showed that lone male chimpanzees often hunt mammals and are sometimes successful (Busse 1978). Observations of chimpanzees hunting and preying upon mammals in nonsocial contexts demonstrate that redirected aggression cannot be an exclusive explanation for chimpanzee hunts.
Teleki (1973) stated that predation and possession of meat may allow young male chimpanzees to enhance their status and that "meat is usually eaten and shared in a leisurely manner more suggestive of pleasure than basic hunger alone" (Teleki 1973, p. 173). Chimpanzees also spend considerable time processing vegetative foods before eating them (Wrangham 1975). Energy and nutrient budgets allocated to different food types have not been measured for primates, but the suggestion that animal food is processed at a leisurely rate relative to vegetative foods is so far unsubstantiated. One basis for Teleki's remarkable (and in our opinion probably erroneous) conclusion is that chimpanzees do gather at kills to compete for a stake. During such events large carcasses are not completely defensible, and fragments incidentally become accessible to subordinates. Shirley Strum4 also witnessed sharing of meat by baboons at large carcasses. In these observations the recipients were not strictly determined by hierarchical status. Long-term associates of several sorts, including related infants and long-term female associates of males, were also tolerated.
Primates carefully process foods that have a relatively indigestible portion. The undigestible bones, fur or feathers, and guts of large prey are carefully removed. Processing intervals allow for recruitment of competitors, who may by various means gain access to a part of a kill.
Micronutrient Hypothesis
This hypothesis is that primates select animal foods to secure essential micro-nutrients, in this case vitamin B12 (Hausfater 1976, Jolly 1972). The idea stems from the observation that vitamin B12 is unavailable in higher plants (Evans and Kliewar 1964). Many captive primate species enter into hypovitaminosis B12 when maintained on vegetarian diets (Huser and Beard 1969, Oxnard 1964, 1966, 1967, Siddons 1974, Siddons and Jacob 1975). However, clinical symptoms of vitamin B12 deficiency take years to develop and are usually not observed earlier than one year following adoption of a vegetarian diet. Similarly, humans who are strictly vegetarian may not develop vitamin B12 deficiency symptoms for several years (Baker 1967, Hines 1966, Smith 1962).
Vitamin B12 is the least readily available vitamin to omnivorous primates. It is the only water-soluble vitamin stored in primate bodies in any quantity, being bound to blood proteins. Primate species vary markedly in the normal serum vitamin B12 level, which may reflect differences in the binding capacity of species-specific vitamin B12-binding proteins (Beard and Huser 1970). Many primates require vitamin B12, but there is no evidence that this need is not secured incidentally in the course of procuring natural diets.
Hausfater (1976) favors the vitamin B12 hypothesis as a basis for primate carnivory. He observed an increase in baboon (Papio cynocephalus) predation upon mammals in the dry season when protein, vitamins, and possibly insect matter were relatively unavailable. But he also noted that mammalian prey were potentially more vulnerable at this season due to reduced cover, so the seasonal increase in predation upon mammals may be attributable to the nutritional hypotheses noted below.
Deficiency diseases have not been identified for any wild primate population (Kerr 1972, Wolf 1972). Known cases of nutritional diseases among primates are laboratory-induced features of captive animals or culturally induced human diseases. Laboratory studies probably cannot establish evidence for the evolutionary basis of dietary needs.
Protein Hypothesis
According to this hypothesis, animal matter is chosen because it is a necessary source of high-quality protein (Dart 1963). Harding (1973) and Hausfater (1976) rejected this hypothesis because plant matter supplies most chimpanzee and baboon dietary protein. Their conclusion probably underestimates the potential importance of animal protein to primates. Although omnivores obtain considerable protein from vegetable matter, its nutritive value is low relative to animal protein (e.g., FAO 1970, Hegsted and Chang 1965, Miller and Bender 1955); i.e., animal matter is generally a more balanced source of amino acids for omnivores than is plant matter. The digestibility of animal protein is also higher than that of plant protein (approx. coefficients of digestibility for animal protein 97%, for fruit protein 85%, for plant protein 60-80%, FAO 1970). Digestibility of foods is inversely related to fiber content, which is negligible for animal matter. Thus, for omnivores, animal matter is a more digestible and higher quality source of protein than plant matter. The critical importance of animal matter in supplying essential amino acids to primates cannot be evaluated from the available evidence.
The dietary significance of human protein-associated deficiency diseases remains uncertain. Here, as for other primates, a central issue is the relationship of calorie to protein deficiency (e.g., Alleyne et al. 1977, McLaren 1974, Olson 1975). Kwashiorkor is no longer regarded as attributable strictly to protein deficiency; in many cases it may be remedied simply by increasing the availability of staple low protein foods (Hegsted 1976, 1978). Since studies of primate nutrition are primitive compared with studies of human nutrition, the relationship of protein to energy needs in primates is unresolved.
Energy Hypothesis
According to this hypothesis, animal matter is chosen for its energy value (Gaulin and Kurland 1976) such that caloric value and food availability determine the dietary choices of omnivorous primates. When availability of animal matter reaches levels where net energy yield per unit foraging time is greater for animal matter than for other food types,
________________
4 S. C. Strum, Anthropology Department, University of California, San Diego, personal communication, 1978.
This hypothesis has been largely overlooked or rejected in earlier discussions of the significance of animal matter to primate diets. By comparison, studies of the food habits of other animal groups (e.g., Pulliam 1974, Pyke et al. 1977, Schoener 1971) suggest that foods are selected to optimize net energy yield per unit foraging time while, incidentally, meeting most or all nutrient requirements. The applicability of this hypothesis to primates can be tested by determining time budgets, calorie intake, and digestibility of specific animal foods relative to other available foods, the focus of our current field studies. Such extensive measurements of energy intake characteristics are unavailable for any primate species.
We favor this energy maximization alternative. The main focus of this comparison of alternative hypotheses is to emphasize the tenuous status of contemporary hypotheses, many of which are regularly cited as complete explanations of why primates eat the kinds of foods they eat.
PRIMATE PREDATION UPON VERTEBRATES
Baboons (Harding and Strum 1976, Hausfater 1976, Strum 1975) and chimpanzees (Lawick-Goodall 1968, Teleki 1973,Wrangham 1975) have received the most attention for their predatory activities, although many other nonhuman primates capture and eat vertebrate prey(Teleki 1975). Baboons commonly prey upon vervet monkeys, hares, and newborn gazelles. Chimpanzees capture bushpigs, bushbuck, and other primates, especially red colobus monkeys (Busse 1977). Predation upon mammals by chimpanzees and baboons is usually opportunistic, i.e., prey animals are encountered at close range and are captured quickly with a high probability of success, a typical scavenge-hunting tactic. For animals securing prey in this way, time and energy costs for searching and pursuing prey are minimized by the opportunistic manner of encounter. This form of carnivory also requires little specialization. Long-range attacks upon large speedy prey species play a smaller part in prey capture by primates.
Chimpanzees and baboons usually consume only vertebrate prey they have killed. Chimpanzees sometimes take fresh kills from baboons (Morris and Goodall 1977), and baboons and chimpanzees regularly take kills from conspecifics. They usually reject fresh carrion (Harding 1973, Lawick-Goodall 1968, Washburn and DeVore 1961), although baboons (Papio anubis) scavenge dead fish from beaches (Oliver 1978) and capture live fish from drying desert pools (Hamilton, personal observations). Shirley Strum5 has observed instances of baboons eating carrion, but the baboons studied by her mainly eat prey items they have killed. Human populations in Africa also tend to avoid eating carrion and dying animals (Fendall and Grounds 1965, Heyworth et al. 1975).
Rejection of carrion and fresh meat by wild and captive primates has led to the unwarranted conclusion that certain or all primates do not prefer or even accept meat. However, there are reasons free-ranging primates should avoid carrion as opposed to live prey, regardless of its dietary value. Anthrax is widespread in Africa (Ebedes 1976), and any anthrax-laden carcass is potentially lethal to primates (Thorpe 1972). Botulism and other diseases are also directly transferable from carcasses to mammals. Thus, there are adaptive reasons to avoid meat unless it is fresh, and this can be guaranteed only by making a kill or by seeing it made. Disease avoidance may explain carrion rejection by primates.
Chimpanzees and baboons usually share access to animal resources only when these resources cannot be defended. Defensible items in short supply are claimed by high-ranking individuals. As individual social rank increases, access to net energy per unit time increases for those foods that can be sequestered individually. Predation by baboons and chimpanzees is primarily an adult male activity. Large prey items captured by females are usually taken away by adult males (Hausfater 1976, Strum 1975, Teleki 1973, Wrangham 1975). Smaller body size and lower social status of females relative to males limit their ability to retain possession of large prey items. Females and juveniles have little to gain by hunting large prey, and they obtain most of their animal matter in the form of invertebrates (McGrew 1978). Low-ranking individuals sometimes are able to consume part of a kill before it can be taken from them by higher-ranking individuals. Chimpanzees often attempt to grab kills from one another and quickly tear apart large carcasses. In this way, subordinate animals sometimes secure part of the kill and flee with it.
At satiation, dominant adult male babons may relinquish parts of carcasses. This is especially true for large prey items such as infant antelope. Such satiation effects may account for the recent expanded participation in meat eating at kills observed by Harding and Strum (1976) in Kenya. Hausfater (1976) found that neonate gazelles (ca. 6 kg) changed hands an average of 4.4 times per carcass. Smaller juvenile vervet monkeys (ca. 3 kg) changed hands 2.3 times, and still smaller hares (ca. 1.5 kg) 1.5 times. These figures include transfer of skull and skin to waiting troop members and emphasize the limited access to kills by troop members in troops averaging 33 individuals. Seventy-three percent of all feeding at kills was accounted for by five adult males (Hausfater 1976).
Under special circumstances primates may share meat of carcasses. Events of mutual sharing by chimpanzees (Teleki 1975, Wrangham 1975) and baboons have been emphasized in reference to primate predation, but these appear to be reports of special circumstances, often among allied individuals. Such observations do have great significance to us in evaluating possible transitions from unshared to shared kills by humans and the development of cooperative hunting.
SIGNIFICANCE TO HUMANS
Diet selection by humans follows the pattern of nonhuman primates: Animal matter generally is chosen when it is available and economical relative to other foods (Greenfield 1974). Animal matter comprises, on the average, 14.8% of the dietary energy for contemporary humans (Statistical Office of the U.N. 1971). This value ranges from 2-3% in several populations to about 40% in the United States and to more than 50% in New Zealand.
Meat is an international status symbol, and 11 “developed” countries consume about 40% of the global meat supply (Greenfield 1974). Internal political decisions are commonly made to provide a larger or cheaper meat supply, and human preference for meat is, thus, central to many contemporary economic and political developments. For example, the recent Russian purchases of large quantities of wheat on the world market were not based upon an internal shortage of wheat for bread production; they were used to feed livestock, thereby sustain-
_______________________
5See footnote 4.
ing the meat-eating proclivities of the Russian populace. Cereal consumption is low in affluent countries, but total utilization is high; a large percentage of this resource is used for livestock feeding (Stamler et al. 1972).
Our analysis can be related to recent human epidemiological studies correlating meat consumption with colon cancer incidence in several countries (Armstrong and Doll 1975, Berg and Howell 1974, Drasar and Irving 1973, Reddy et al. 1975). High meat diets tend to be low in fiber content; this is significant since fiber-deficient diets may play an etiologic role in colon carcinogenesis (Modan et al. 1975), although this is still a controversial topic (Stavraky 1976).
Meat consumption is implicated also in connection with atherosclerosis (e.g., see Stamler et al. 1972). Animal fats and animal protein fed in large quantities to captive primates have atherogenic effects (e.g., see Strong 1976, Strong et al. 1976). Here, as in the case of colon cancer, the importance of dietary risk factors may be influenced by the type and amount of fiber in the diet (Kritchevsky et al. 1977). These studies do not demonstrate a causal diet-disease relationship, but they do suggest that the human digestive system is poorly adapted to some contemporary luxury diets, especially diets equivalent to ad libitum cafeteria choices offered to animals to determine food preferences. Supermarkets, available to many contemporary human populations, are a similar measure of food choice in the presence of nutritionally nearly unlimited alternatives. These preferences in contemporary subsistence economies sometimes are restrained by lack of availability of certain preferred foods. Relaxation of economically limiting conditions for some contemporary human populations may have deleterious consequences.
The human propensity to expand dietary meat consumption seems to be a legacy from our omnivorous primate heritage. Humans apparently share with most primates a tendency to increase the proportion of dietary animal matter whenever it is economical to do so. We inherited dietary preferences for animal matter, which historically have been limited by economics and a hierarchical society. Under current luxury diet circumstances, no such balance of diet to resource availability prevails and preference betrays best interest. Only religious beliefs or perception of the health hazards of high meat diets will inhibit those with access to luxury diets from developing and maintaining meat consumption at health-hazard levels only briefly attainable by our primate predecessors and hominid ancestors.
ACKNOWLEDGMENTS
We are indebted to A. Clark, R. O. Davis, B. Hill, P. J. Richerson, R. C. Roberts, R. A. Tilson, and D. S. Wilson for comments and discussion of the manuscript. B. Hill assisted in data analysis. We are especially indebted to P. C. Arrowood for her generous help on all aspects of this project.
Permission to work in the Namib Desert was provided by B. J. G. De la Bat, Director, Nature Conservation, Administration, South West Africa/Namibia, and in Botswana by E. T. Matenge, Director, Department of Wildlife, National Parks and Tourism. Our analysis was supported by NSF grant no. GB2853X2 and NIH grant 1 R01 RR01078-01 and by a University of California Chancellor's Graduate Fellowship to C. Busse.
REFERENCES CITED
Alleyne, G. A., R. W. Hay, D. I. Picour, J. P. Stanfield, and R. G. Whitehead. 1977. Protein-Energy Malnutricion. Edward Arnold, London.
Armstrong, B., and R. Doll. 1975. Environmental factors and cancer incidence and mortality in different countries, with special reference to dietary practices. Int. J. Can-cer. 15: 617-631.
Baker, S. J. 1967. Human Vitamin B12 deficiency. World Rev. Nutr. Diet. 8: 62-126.
Bauchop, T. 1971. Stomach microbiology of primates. Annu. Rev. Microbiol. 25: 429-436.
Bauchop, T., and R. Martucci. 1968. Ruminant-like digestion of the langur monkey. Science 161: 698-700.
Beard, M. E. J., and H. J. Huser. 1970. Studies on folate and Vitamin B12 metabolism in primates. II. Vitamin B12 binding proteins. Folia Primatol. 12: 305-312.
Berg, J. W., and M. A. Howell. 1974. The geographic pathology of bowel cancer. Cancer 34: 807-814.
Busse, C.D. 1977. Chimpanzee predation as a possible factor in the evolution of red colobus monkey social organization. Evolution 31: 907-911.
______. 1978. Do chimpanzees hunt cooperatively? Am. Nat. 112: 767-770.
Charles-Dominique, P. 1974. Ecology and feeding behaviour of five sympatric lorisids in Gabon. Pages 131-150 in R. D. Martin, G. A. Doyle, and A. C. Walker, eds. Prosimian Biology. University of Pittsburgh Press, Pittsburgh, PA.
Chivers, D. J. 1972. The siamang and gibbon in the Malay Peninsula. Pages 103-135 in D. M. Rumbaugh, ed. Gibbon and Siamang, Vol. 1. Karger, Basel.
Chivers, D. J., J. J. Raemaekers, and F. P. G. Aldrich-Blake. 1975. Long-term observations of siamang behaviour. Folia Primatol. 23: 1-49.
Clutton-Brock, T. H. 1975. Feeding behaviour of red colobus and black and white colobus in East Africa. Folia Primatol. 23: 165-207.
Clutton-Brock, T. H., and P. H. Harvey. 1977. Primate ecology and social organization. J. Zool. 183: 1-39.
Crile, G., and D. P. Quiring. 1940. A record of the body weight and certain organ and gland weights of 3690 animals. Ohio J. Sci. 40: 219-259.
Dart, R. A. 1963. The carnivorous propensity of baboons. Symp. Zool. Soc. London 10: 49-56.
Drasar, B. S., and D. Irving. 1973. Environmental factors and cancer of the colon and breast. Br. J. Cancer 27: 167-172.
Dunbar, R. I. M. 1977. Feeding ecology of gelada baboons: a preliminary report. Pages 251-273 in T. H. Clutton-Brock, ed. Primate Ecology. Academic Press, New York.
Dunbar, R. I. M., and E. P. Dunbar. 1974. Ecological relations and niche separation between sympatric terrestrial primates in Ethiopia. Folia Primatol. 21: 36-60.
Ebedes, H. 1976. Anthrax epizootics in wildlife in the Etosha National Park, South West Africa. Pages 519-526 in L. A. Page, ed. Wildlife Diseases. Plenum Publishing Co., New York.
Evans, H. J., and M. Kliewar. 1964. Vitamin B12 compounds in relation to the require requirements of cobalt for higher plants and nitrogen-fixing organisms. Ann. N. Y. Acad. Sci. 112: 735-755.
FAO. 1970. Amino-acid content of foods and biological data on proteins. Food and Agriculture Organization, Nutritional Studies No. 24, Washington, DC.
Fendall, N. R. E., and J. G. Grounds. 1965. Incidence and epidemiology of disease in Kenya. I. Some diseases of social significance. J. Trop. Med. Hyg. 68: 77-84.
Gaulin, S. J. C., and J. A. Kurland. 1976. Primate predation and bioenergetics. Science 191: 314-317.
Gautier-Hion, A., and J. P. Gautier. 1974. Les associations polyspecifiques de Cercopitheques du Plateau de M'possa (Gabon). Folia Primatol. 22: 134-177.
Greenfield, J. N. 1974. Effect of price changes on the demand for meat. Mon. Bull. Agric. Econ. Stat. 23(12): 1-8.
Hamilton, W. J. III. 1973. Life's Color Code. McGraw-Hill, New York.
Hamilton, W. J. III, R. E. Buskirk, and W. H. Buskirk. 1978. Omnivory and utilization of food resources by chacma baboons. Am. Nat., in press.
Harding, R. S. O. 1973. Predation by a troop of olive baboons (Papio anubis). Am. J. Phys. Anthrop. 38: 587-592.
Harding, R. S. O., and S.C. Strum. 1976. The
predatory baboons of Kekopey. Nat. Hist. 85: 46-53.
Hausfater, G. 1976. Predatory behavior of yellow baboons. Behaviour 56: 44-68.
Hegsted, D. M. 1976. Protein needs and possible modifications of the American diet. J. Am. Diet. Assoc. 68: 317-320.
_____. 1978. Protein-calorie malnutrition. Am. Sci. 66: 61-65.
Hegsted, D. M., and Y. Chang. 1965. Protein utilization in growing rats. I. Relative growth index as a bioassay procedure. J. Nutr. 85: 159-168.
Heyworth, B., M. E. Ropp, U. G. Voos, H. I. Meinel, and H. M. Darlow. 1975. Anthrax in the Gambia: an epidemiological study. Br. Med. J. 4: 79-82. .
Hill, W. C. O. 1970. Primates: Comparative Anatomy and Taxonomy. Wiley, New York.
Hill, W. C. O., and R. E. Rewell. 1948. The caecum of primates: its appendages, mesenteries and blood supply. Trans. Zool. Soc. Lond. 26: 199-258.
Hines, J. D. 1966. Megaloblastic anemia in an adult vegan. Am. J. Clin. Nutr. 19: 260-268.
Hladik, C. M. 1977. A comparative study of the feeding strategies of two sympatric species of leaf monkeys: Presbytis senex and Presbytis entellus. Pages 324-353 in T. H. Clutton-Brock, ed. Primate Ecology. Academic Press, New York.
Huser, H. J., and M. E. J. Beard. 1969. Studies on folate and vitamin B12 metabolism in primates. I. Blood and bone marrow morphology, folate, and vitamin B12 levels. Folia Primatol. 10: 172-180.
Jolly, A. 1966. Lemur Behavior. University of Chicago Press, Chicago, IL.
_____. 1972. The Evolution of Primate Behavior. Macmillan, New York.
Kerr, G. R. 1972. Nutritional requirements of subhuman primates. Physiol. Rev. 52: 415-467.
Klein, L. L., and D. B. Klein. 1977. Feeding behaviour of the Colombian spider monkey. Pages 153-181 in T. H. Clutton-Brock, ed. Primate Ecology, Academic Press, New York.
Kortlandt, A. 1972. New Perspectives on Ape and Human Evolution. Stichting voor Psychobiologie, Amsterdam, the Netherlands.
Kritchevsky, D., S. A. Tepper, D. E. Williams and J. A. Story. 1977. Experimental atherosclerosis in rabbits fed cholesterol-free diets. Part 7. Interaction of animal or vegetable protein with fiber. Atherosclerosis 26: 397-403.
Lawick-Goodall, J. van. 1968. The behaviour of free-living chimpanzees in the Gombe Stream Reserve. Anim. Behav. Monogr. 1: 161-311.
MacKinnon, J. 1974. The behaviour and ecology of wild orang-utans (Pongo pygmaeus). Anim. Behav. 22: 3-75.
McGrew, W. C. 1978. Evolutionary implications of sex differences in chimpanzee predation and tool use. In D. A. Hamburg, L. McGowan and J. Goodall, eds. Perpectives on Human Evolution IV. The Behaviour of Great Apes. Staples/Benjamin, Menlo Park, CA, in press.
McLaren, D. S. 1974. The great protein fiasco. Lancet ii: 93-96.
Miller, D. S., and A. E. Bender. 1955. The determination of the net utilization of proteins by a shortened method. Br. J. Nutr. 9: 382-383.
Modan, B., V. Barell, F. Lubin, M, Modan, R. Greenberg and S. Graham. 1975. Low fiber intake as an etiologic factor in cancer of the colon. J. Natl. Cancer Inst. 55: 15-18.
Morris, K., and J. Goodall. 1977. Competition for meat between chimpanzees and baboons of the Gombe National Park. Folia Primatol. 28: 109-121.
Napier, J. R., and P. H. Napier. 1967. A Handbook of Living Primates. Academic Press, London.
Nishida, T. 1968. Social behavior and relationship among wild chimpanzees of the Mahali Mountains. Primates 11: 47-87.
Oliver, J. 1978. Feeding behaviour of olive baboons, Papio anubis, at Gombe National Park, Tanzania. Ph.D. Thesis, University of Cambridge.
Olson, R. E., ed. 1975. Protein-Calorie Malnutrition. Academic Press, New York.
Oxnard, C. E. 1964. Some variations in the amount of vitamin B12 nutrition in some primates in captivity. Nature (Lond.) 201: 1188-1191.
_____. 1966. Vitamin B12 nutrition in some primates in captivity. Folia Primatol. 4: 424-431.
_____. 1967. Some occult lesions in captive primates. Am. J. Phys. Anthrop. 26: 93-96.
Pulliam, R. H. 1974. On the theory of optimal diets. Am. Nat. 108: 59-74.
Pyke, G. H., H. R. Pulliam, and E. L. Charnov. 1977. Optimal foraging: a selective review of theory and tests. Q. Rev. Biol. 52: 137-154.
Quris, R. 1975. Ecologie et organisation sociale de Cercocebus galeritus agilis dans le Nord-est du Gabon. Terre Vie 29: 337-398.
Reddy, B. S., J. H. Weisburger, and E. L. Wynder. 1975. Effects of high risk and low risk diets for colon carcinogenesis on fecal microflora and steroids in man. J. Nutr. 105: 878-885.
Richard, A. 1974. Intra-specific variation in the social organization and ecology of Propithecus verreauxi. Folia Primatol. 22: 178207.
Rodman, P. S. 1977. Feeding behaviour of orang-utans of the Kutai Nature Reserve, East Kalimanton. Pages 384-413 in T. H. Clutton-Brock, ed. Primate Ecology. Academic Press, New York.
Schoener, T. W.. 1971. Theory of feeding strategies. Annu. Rev. Ecol. Syst. 2: 369404.
Schultz, A. H. 1941. The relative size of cranial capacity in primates. Am. J. Phys. Anthrop. 28: 273-287.
Siddons, R. C. 1974. The experimental production of vitamin B12 deficiency in the baboon (Papio cynocephalus). A two year study. Br. J. Nutr. 32: 219-228.
Siddons, R. C., and F. Jacob. 1975. Vitamin B12 nutrition and metabolism in the baboon (Papio cynocephalus). Br. J. Nutr. 33: 415424.
Smith, A. D. M. 1962. Veganism: a clinical survey with observations on vitamin B12 metabolism. Br. Med. J. 1: 1655.
Stamler, J., D. M. Berkson, and H. A. Lindberg. 1972. Risk factors: their role in the etiology and pathogenesis of the atherosclerotic diseases. Pages 41-119 in R. W. Wissler and J. C. Geer, eds. The Pathogenesis of Atherosclerosis. Williams and Wilkins, Baltimore, MD.
Statistical Office of the United Nations. 1971. Statistical Yearbook 1970. United Nations Organization, New York.
Stavraky, K. M. 1976. The role of ecologic analysis in studies of the etiology of disease: a discussion with reference to large bowel cancer. J. Chronic Dis. 29: 435-444.
Strong, J. P., ed. 1976. Atherosclerosis in Primates. Karger, Basel.
Strong, J. P., D. A. Eggen, and S. K. Jirge. 1976. Atherosclerotic lesions in baboons by feeding an atherogenic diet for four years. Exp. Mol. Pathol. 24: 320-332.
Struhsaker, T. T. 1975. The Red Colobus Monkey. University of Chicago Press, Chicago, IL.
Strum, S. C. 1975. Primate predation: interim report on the development of a tradition in a troop of olive baboons. Science 187: 755757.
Suzuki, A. 1971. Carnivory and cannibalism observed among forest-living chimpanzees. J. Anthrop. Soc. Nippon. 79: 30-48.
Teleki, G. 1973. The Predatory Behavior of Wild Chimpanzees. Bucknell University Press, Pennsylvania.
_____. 1975. Primate subsistence patterns: collector-predators and gatherer-hunters. J. Hum. Evol. 4: 125-184.
Thorpe, B. D. 1972. Anthrax, fusiformis, anaerobic organisms. Pages 335-380 in R. N. T-W-Fiennes, ed. Pathology of Simian Primates. Karger, Basel.
Washburn, S. L., and I. DeVore. 1961. Social behavior of baboons and early man. Pages 91-105 in S. L. Washburn, ed., Social Life of Early Man. Aldine, Chicago, IL.
Waser, P. 1975. Monthly variations in feeding and activity patterns of the mangabey, Cercopithecus albigena (Lydekker). E. Afr. Wildl. J. 13: 249-263.
Wolf, R. H. 1972. The pathology of nutritional defciencies in non-human primates. Pages 239-269 in R. N. T-W-Fiennes, ed. Pathology of Simian Primates. Karger, Basel.
Wrangham, R. W. 1975. The behavioural ecology of chimpanzees in Gombe National Park, Tanzania. Ph.D. Thesis, University of Cambridge.
Yoshiba, K. 1968. Local and intertroop variability in ecology and social behavior of common Indian langurs. Pages 217-242 in P. Jay, ed. Primates. Studies in Adaptation and Variability. Holt, Rinehart and Winston, New York.
BioScience Vol. 28 No. 12